The difference is in the material and how it’s produced. The carbon electrode is amorphous, composed of a mixture of carbonaceous materials and baked at approximately 1400 degree celsius while the graphite electrode is artificial; made by graphitizing carbon electrode at higher than 3000 degree celsius that realigns the element into a graphite electrode lattice structure.
They differ in their architecture and that’s what makes them work. Graphite electrodes have better properties, including high electrical and thermal conductivity, high mechanical strength, and high resistance to thermal shock. This enables them to work at higher current densities and temperatures, so they are the only standard in modern EAF steelmaking that accounts for most of the worldwide production.
Non-pure carbon electrodes, Some carbon electrodes referred to sometimes as “amorphous carbon” are not as pure, brittle and have significantly greater electrical resistance. Their primary benefit is cost, which is much less. Smaller plants require water for less intensively cooled applications such as ferro alloys production, electric arc furnaces for the production of non-ferrous metals e.g Pb and Zn, and specific types of electrolysis. Graphite is the top quality alternative for aggressive industrial applications, carbon electrodes are a value choice for less demanding applications.
Graphite Electrodes:
The Graphite Electrodes are conductive materials used in electric furnace (EAF) steel production that serves as a melting agent. They are massive, cylindrical blocks of expensive petroleum needle coke processed in an expensive but very complex and protracted process known as graphitization by high temperatures close to 3000 degree celsius. This yields synthetic material with an overall relatively regular crystalline structure.
Among their characteristics are excellent thermal stability, good electrical conductivity, mechanical strength to stand the harsh environments of a furnace. They carry the vast electrical power needed to produce an arc of over 3000 degree celsius to melt and recycle scrap metal. Thermal shock resistance helps prevent the glass from shattering when subjected to rapid temperature changes.
Besides the recycling of steel, they play a key role in the refinery of other metals such as silicon and phosphorus and in the production of non-ferrous metals in electric furnaces. Due to their low electrical electrical resistance and high oxidation resistance, the performance thereof has and can have a direct effect on the melting efficiency, energy consumption and operating cost and thus they are a “vital – – consumable” in modern day industrial metallurgy.
Carbon Electrodes:
Carbon Electrodes, commonly referred to as “amorphous carbon” electrodes are conductive elements manufactured from a mixture of carbon-containing materials such as petroleum coke, coal tar pitch and anthracite. They are produced by blending, shaping and partial baking at temperatures 1200-1400 degree celsius. This carbonizes the binder without producing a crystalline graphite structure in the material and so this is referred to as “amorphous”.
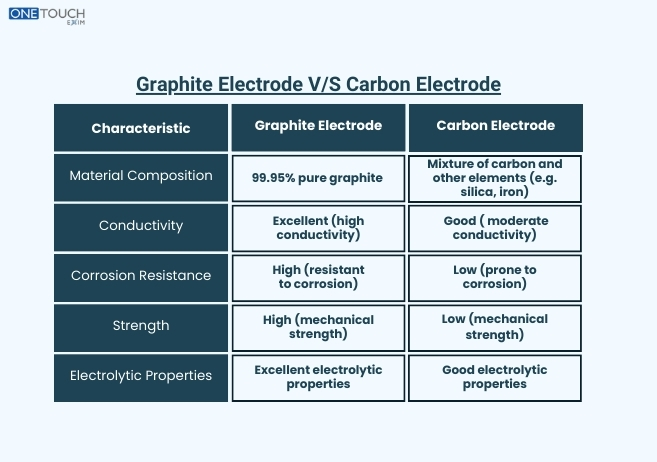
The important properties of the C/C-composites are good mechanical strength and chemical resistance, which are superior compared to graphite, but electrical resistivity and thermal conductivity are higher and lower, respectively. They are also more fragile and less resistant to thermal shock.
These benefits enable their use in less demanding electrochemical and metallurgical processes. Where high temperatures are not required. They are often used in arc furnaces, as well as to fuel anode baking furnaces and to produce ferroalloys and metal in several different types of smelting operations, with the largest application being in copper smelting and ferrous-silicon production for the steel industry. They are a less expensive alternative to graphite electrodes for some industrial applications where the higher quality of graphite is unnecessary.
Comparison Between Graphite Electrode and Carbon Electrode
The fundamental difference is in their atomic makeup and how they are made. Carbon electrodes are “amorphous” and heated solid to 1400 degree celsius and graphite electrodes 3000 degree celsius to produce an ordered crystalline lattice.
This leads to higher performance for graphite electrodes. They show good electrical and thermal conductivity, mechanical strength and thermal shock resistance. This enables them to be used under the harsh temperatures and the high current densities of the moder EAF steelmaking (where they find their main applications).
Carbon electrodes are less ductile, have higher electrical resistance and lower thermal conductivity. Their primary advantage is being much cheaper. They are employed in less robust applications, some in ferroalloy production, non-ferrous metal melting and electrolytic cells such as those for smelting of aluminium.
The curve in question is application based. High performance requires premium Graphite electrodes for ultra high temperatures arc furnaces. Carbon electrodes have been used as an economic alternative for lower-current applications where their poorer electrical conductivity has not been a first order constraint.
Key Differences of Graphite Electrodes and Carbon Electrodes:
Graphite and carbon electrodes are characterized by how they are made, their material characteristics and their end uses.
Composition & Structure:
Carbon electrodes are “amorphous” , a disordered mix of carbon materials basked at 1400 degree celsius graphite electrodes are synthetic-ally graphitized at 3000 degree celsius, giving it an extremely ordered, crystalline atomic structure.
Performance Features:
Graphite has a highly anisotropic and crystalline structure to provide much higher electrical and thermal conductivity, higher mechanical strength and good thermal shock resistance. The carbon electrodes of a higher electrical resistivity are more fragile and have the potential for fracture upon rapid temperature cycling.
Main Applications:
Our graphite electrodes are required as conductive materials in various high temperatures and intensive operations in EAF steel industry. Carbon electrodes are also used in some less common manufacturing, such as certain types of ferroalloy production, nonferrous smelting and in the manufacture of phosphorus.
Note :- Graphite is the high performance option for the industrial application and carbon electrodes work efficiently with lower intensity applications or requirements for economical solutions. The decision depends upon the current Density, Temperature, Operating Cost.
Conclusion
The selection of graphite and carbon electrodes depends on application demand and cost. Graphite is excellent in the high temperature, high-conductivity process of EAF steelmaking. For lower duty applications such as production of ferroalloys a carbon electrode can be a less expensive alternative. ochemical experiments and electrolysis due to their conductivity and ease of electron transfer. This comparison highlights the importance of understanding the unique characteristics of graphite and carbon electrodes to optimize their usage in different industrial settings.