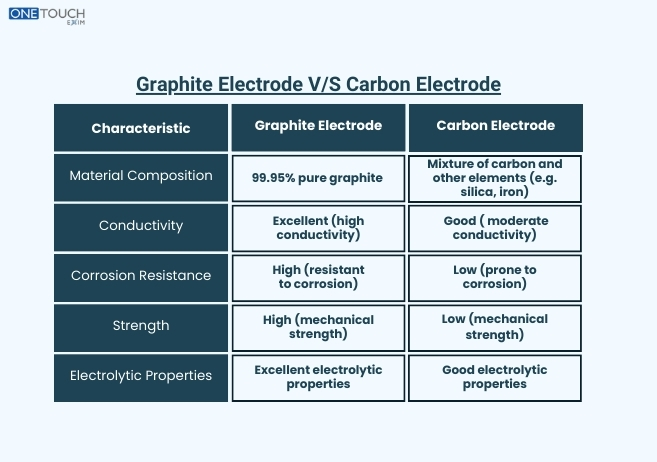
Graphite electrodes and Carbon electrodes are the two most common electrodes used in various sectors or applications. In industrial processes and manufacturing, the electrodes plays an important role in applications such as electric arc welding, electromechanical reactions, and arc furnaces. Graphite and carbon electrodes are similar to each other but they have different properties based on performance suitability for specific tasks. Let us discuss the key difference between graphite electrodes and carbon electrodes, their characteristics and differences.
Graphite Electrodes:
These electrodes are made up of high-quality graphite materials. These are popularly known for their high melting point, low thermal expansion properties, and exceptional conductivity. Graphite electrodes are used in various applications that involve high-temperature environments such as in steel production for electric arc furnaces, where they contribute as conductive elements and produce intense heat through electric arcs.
The main and most important advantage of graphite electrodes is the ability to stand in extremely high temperatures without losing their structural integrity or deforming. Based on this advantage or property this is the most preferable electrode used in processes where prolonged exposure to intense heat is required for melting and refining of metals.
Graphite electrode has an excellent electrical conductivity that allows or is used for efficient transmission of electric current through the material of electrodes. The property of having high conductivity is important in applications like arc welding because in arc welding the electrode must have the amount of current to perform a stable arc.
Another important key factor of graphite electrodes is corrosion resistance. It resists chemical reactions and corrosion. It has a high amount of chemical inertness that makes it the best option for welders to use in reactive substances or corrosive agents environments.
There are also limitations of graphite electrodes. The main factor or drawback of these electrodes is they are more expensive as compared to other rods. The prices of graphite electrodes are high because of the use of premium graphite materials.
Carbon Electrodes:
These electrodes are made up of a combined mixture of carbon and other materials such as petroleum coke, carbon black, and coal tar pitch. As compared to graphite electrodes, carbon electrodes are less resistant to high temperatures, thermal shocks, and lower purity levels. That is why they are only used in applications that do not need the same level of thermal stability as compared to graphite electrodes.
The key advantage of carbon electrodes is their cost-effectiveness and affordability. Carbon electrodes are more options economically for businesses reducing their expenses because of their simple manufacturing and lower-cost raw materials used in the production.
Moreover, carbon electrodes are more easier and flexible to shape as compared to graphite electrodes which makes them suitable for those applications that require custom electrode designs and intricate. The softer characteristics and lower density allow easier machining that can be more beneficial for electrode customization.
Under extreme heat conditions, carbon electrodes oxidize or deform more quickly leading to the shorter lifespan of electrodes and performance issues in industrial environments. The stability and lower thermal conductivity of carbon electrodes can limit their appropriateness in certain high-temperature projects such as electric arc welding and steel production.
Comparison Between Graphite Electrode and Carbon Electrode
Both graphite and carbon electrodes are important materials that are used in different industrial projects, especially in electrolysis and steelmaking. They have similarities and distinct characteristics which make them suitable for use in different applications.
| Graphite Electrodes | Carbon Electrodes |
| It is a good conductor of electricity because it has carbon atoms arranged in hexagonal patterns. | These electrodes are made up of anthracite, and metallurgical coke and are used in manufacturing processes |
| Graphite electrodes are manufactured using high-quality refractory raw materials. | They are easy to handle and more flexible |
| Because of conductivity and durability, it has been used in electric furnaces’ steelmaking processes. | They are used in electrochemical analysis and experiments, where electron transfer is crucial for successful electrolysis. |
| Well known for their high conductivity, stability at high temperatures, and durability. | They are good conductors of electricity and important for projects having the requirements of transfer of electrons. |
Key Differences of Graphite Electrodes and Carbon Electrodes:
Structure:
Graphite electrodes have a layered or organized structure that allows the movement of electrons, whereas carbon electrodes are rod-shaped and resemble pencil leads.
Conductivity:
Both graphite and carbon electrodes are good conductors of electricity. Graphite electrodes are preferred for their high conductivity because of their delocalized electrons in the structure.
Applications:
Graphite electrodes are used in steelmaking while carbon electrodes are used in applications such as electrolysis processes and electrochemical analysis.
Durability:
Graphite electrodes are well known for their durability and longevity which makes them suitable for long-lasting use in various applications whereas carbon electrodes have minimized downtime, longevity, lower maintenance cost, and consistent performance.
Conclusion
In conclusion, while both graphite and carbon electrodes serve as conductive materials, their distinct properties make them suitable for specific applications. Graphite electrodes excel in high-temperature processes like steelmaking, thanks to their superior conductivity and durability. On the other hand, carbon electrodes are more flexible and find their niche in electrochemical experiments and electrolysis due to their conductivity and ease of electron transfer. This comparison highlights the importance of understanding the unique characteristics of graphite and carbon electrodes to optimize their usage in different industrial settings.